Rework and scrap pose critical challenges to manufacturers, particularly in industries requiring precision and reliability. Defective or out-of-spec components lead to increased costs, production delays, and strained supplier relationships. For safety-critical components in highly regulated industries like aerospace and automotive, these issues are especially damaging, creating bottlenecks that disrupt the supply chain and delay deliveries to OEMs.
The Medical, Automotive, Aerospace, Military and RF/Microwave Industries, regardless of their unique functions, often demand exceptional conductivity. Electro-Spec intends to meet the demands which sophisticated electrical components require.
Products Finishing magazine names Electro-Spec to the ‘Top Shops” list
CINCINNATI, Ohio – Electro-Spec, Inc. has been named one of the best finishing shops in the U.S., according to an industry benchmarking survey conducted by Products Finishing magazine, a trade publication which has covered the industry since 1938.
Accounting for Plating Thickness in Assembly Design
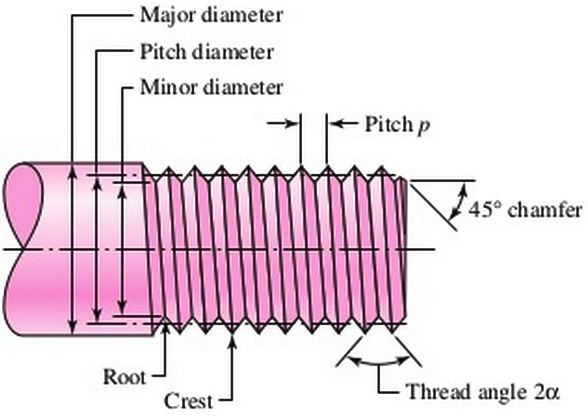
The main consideration when plating threaded components would be to ensure that the plated threads properly fit into their mated counterparts. As shown in Figure 1-1, the dimensions to consider here would be the diameter and pitch of the threads. This translates into the tolerance of the plated coating being accounted for within the assembly because the plated coating will slightly change the geometry of the threaded component. The thickness of the plating should be accounted for when the part is designed. The most ideal location to account for the plated coating would be within the specifications of the mating part, including diameter and pitch. Of course, certain calculations are required to determine the slight change in the diameter or some other dimension of the mated part which will not impact the overall strength of the assembly. But, this is a calculation that should be performed before arbitrarily designating a plating thickness.
Precious metal plating is an expensive process, especially because the standard method for improving corrosion is to increase the plating thickness. With self-assembled molecules (SAM’s), however, metal plating can be reduced significantly without losing corrosion protection. In addition to enhanced corrosion protection, SAM’s will also allow plated components to have increased conductivity.
Conductivity and Reduced Metal Thickness
Precious metal plated items, and in fact any plated item, experience increased resistivity because of uneven surfaces. This resistivity becomes even worse when the plating is thicker. Most manufacturers are forced to increase the thickness of the plating to stop corrosion, but this can decrease the performance of the item while increasing the cost of production. SAM’s addresses both of these issues. The molecular shield provided through SAM’s helps slow the corrosion process down and allows manufactures to use a thinner plating deposit.
When this happens, the conductivity of the plated item improves as well. This is because the plating is thinner and the molecular shield provides a more even surface that lowers resistance. Contact resistance due to uneven surfaces caused by thicker plating creates localized heating. With SAM’s, however, this problem is solved because the molecular shield fills the voids in any uneven surfaces of the plated component. The surface then becomes smoother, enhancing conductivity.
Ever the innovator, Electro-Spec’s engineering team identified a need for a plating process that would enhance the performance of RF connectors. What they developed was the TRI-M3™ finish; this cutting edge process represents a dramatic improvement in plating RF connectors.
The TRI-M3™ process provides high corrosion and tarnish resistance with a shelf life that far exceeds traditional silver plating, and with superior electrical performance to nickel. This plating method overcomes the negative characteristics of silver and nickel plating, while enhancing their positive properties. Silver exhibits excellent electrical properties, but tends to tarnish and break down over time, while nickel exceeds silver regarding longer shelf life and wear characteristics, but exhibits poor intermodulation performance, high permeability, and poor screening effects.