When your engineering prints call for "nickel plating" without specifying the chemistry, you're leaving critical performance decisions to interpretation. The choice between matte (sulfamate) and bright (sulfate) nickel isn't only about cosmetics. It's an engineering decision that impacts mechanical properties, coverage uniformity, and long-term reliability in ways that can make or break your application's performance.
For engineers working with mission-critical parts, boreholes, inner diameters (IDs), and tapped holes are often where performance lives or dies. But when it comes to plating these internal features, even small design decisions can have outsized consequences for quality, cost, and timeline.
When it comes to high-reliability components, plating isn’t just the finishing touch—it’s a critical performance factor. But for parts with complex geometries, achieving consistent, functional, and spec-compliant plating can be far from simple.
The Medical, Automotive, Aerospace, Military and RF/Microwave Industries, regardless of their unique functions, often demand exceptional conductivity. Electro-Spec intends to meet the demands which sophisticated electrical components require.
Electroplating is a process whereby one metal is plated onto another via an electrodeposition method. Customers seek out electroplating for their parts for many reasons such as aesthetics, corrosion protection, increased hardness, wear resistance, increased conductivity, and decreased friction. It allows manufacturers to use base metals that are less expensive and apply a high quality coating to them to achieve the certain desired properties on the finished part.
Electro-Spec has been providing award winning electroplating and electroless plating services to customers for over five decades. This includes applications for lifesaving and safety critical components. Plating is available in precious and semi-precious materials including gold, silver, nickel, copper, Tri-M3TM (Tri-Alloy), electroless and electrolytic nickel. This short article discusses these plating options, as well as their benefits and examples of industries that they are often found of use within.
Products Finishing magazine names Electro-Spec to the ‘Top Shops” list
CINCINNATI, Ohio – Electro-Spec, Inc. has been named one of the best finishing shops in the U.S., according to an industry benchmarking survey conducted by Products Finishing magazine, a trade publication which has covered the industry since 1938.
Accounting for Plating Thickness in Assembly Design
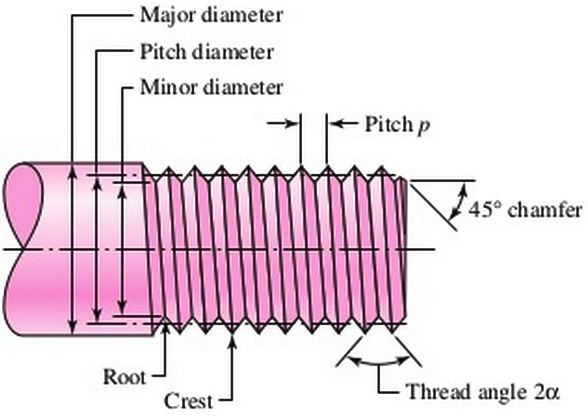
The main consideration when plating threaded components would be to ensure that the plated threads properly fit into their mated counterparts. As shown in Figure 1-1, the dimensions to consider here would be the diameter and pitch of the threads. This translates into the tolerance of the plated coating being accounted for within the assembly because the plated coating will slightly change the geometry of the threaded component. The thickness of the plating should be accounted for when the part is designed. The most ideal location to account for the plated coating would be within the specifications of the mating part, including diameter and pitch. Of course, certain calculations are required to determine the slight change in the diameter or some other dimension of the mated part which will not impact the overall strength of the assembly. But, this is a calculation that should be performed before arbitrarily designating a plating thickness.
Given the importance of the plating process and the value of the material being used, it would be logical to assume that parts would be designed for the most efficient and effective electroplating possible. Unfortunately due to intricate engineering designs and micro miniature components, it is not always feasible to have a “plating friendly” part.
Electroplating, along with other finishing options, take their place at the end of the manufacturing process. It is incumbent on all parties, including the plater to work together in order to provide the best component possible.
Additionally, sometimes a part’s design will necessitate a difficult structure for applying even finishing, whether it’s electroplating or a more traditional finishing method. There are a myriad of reasons why parts are created with geometries that make them difficult to electroplate.
At its heart, passivation is a process that helps prevent corrosion and pitting on the surface of stainless steel. The passivation process applies a thin transparent passive chemically inert film to stainless steel that reduces the reactivity of the metal. This film deters corrosion, oxidation, and mild chemical attack.