Know your process and requirements for durable, high conductivity, corrosion resistant plating.
Metal finishing has transformed itself within a few decades from what was once an empirical craft into a key technology grounded on scientific principles. 1
Modern electroplating is a form of metal finishing used in various industries, including aerospace, automotive, military, medical, RF microwave, space, electronics and battery manufacturing. It is the electrochemical process whereby metal ions in solution are bonded to a metal substrate via electrodeposition.
Prior to electroplating, parts must be cleaned and follow a process of chemical baths to prepare, or activate, them such that a strong bond, and therefore strong adhesion, is created during the electrodeposition process.
The electroplating bath involves many variables and components which must be closely monitored. A power supply provides a flow of direct current to the parts and the electrical connections at the plating bath. This flow of current initiates the attraction of ions in the solution to the surface of the metallic part.
For every mole of electrons that is transferred to the part, one mole of metallic ions in solution will adhere to the part. In addition, a chemical reaction occurs at the surface of the part, involving the reduction and oxidation of ions.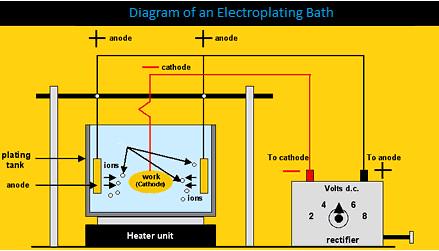
(Photo courtesy of The Time Preserve/watchplating.com)
What an Engineer or Designer Should Consider Before Electroplating
- Nesting of parts during the electroplating process. Since electroplating involves both an electrical and a chemical reaction at the surface of the part, exposure to the plating chemistry is critical to the overall performance of the finished product. Nesting of parts will result in a lack of adhesion or coverage on the surface of the finished part.
- The tolerance on critical part dimensions should be designated with the plating thickness in mind. This also means that the fit into the overall assembly should be accounted for.
- The environment that the finished parts will be exposed to. This will help to determine the plating thickness required for a part to be resistant to corrosion or repeated cycles of wear, for example.
- Because electroplating involves the use of current to initiate a reaction at the surface of a part, the overall geometry of the part will influence the current distribution, often called current density, across the surface of a part. Plating tends to build up on features such as sharp corners, bends or threads. Advanced plating processes exist that can prevent this issue from occurring.
- Drainage of plating solution (surface preparation or plating bath chemistries) such that the inside surfaces of parts will be sufficiently covered and the plating will have adequate bond strength. For some parts, this means the addition of a weep hole during the design phase.
- The intended use and required characteristics (e.g., conductivity, low friction, high strength and resistance to corrosion, wear, etc.). These criteria should be sufficient in designating the type of metal that should be used for finishing each specific part.
**
What Are the Benefits of Electroplating?**
Electroplating enhances or alters the properties of a metallic part.
Depending on the use of the part, a manufacturer may want better wear and abrasion resistance, protection from corrosion, greater lubricity and lower friction, improved EMI/RFI shielding, temperature and impact resistance, improved conductivity, improved solderability, reduced porosity, added hardness or strength or to build up thickness on small or undersized parts.
In addition to the mechanical or functional properties which may be altered during the electroplating process, often the overall aesthetics of the finished part are also important.
**
Types and Methods of Electroplating**
Specialty plating facilities can plate different base materials utilizing various surface finishes:
Common Base Materials
|
|
|
| --- | --- |
Common Surface Finishes
|
|
|
| --- | --- |
The plating material, plating method and parts to be plated will all vary depending on the application.
Gold plating provides excellent electrical conductivity, making it one of the best choices for electrodes, current carrying pins and circuit board components. Gold is ideally suited for protection against intense heat and corrosion in a wide range of environments and climates.
Silver plating is also often used for electronics (over a copper “flash”) due to its lower electrical resistance.
Nickel plating is common because it offers superior chemical and corrosion resistance along with greater wear resistance, which increases product lifecycles. Nickel can be a substitute for silver in electronics or can be used as a coating on steel for an alternative to products made of more expensive stainless steel. Nickel also offers a bright surface finish that can be adjusted according to customer specifications.
Copper plating is typically utilized as a plating layer before the final layer of metal is deposited. This surface finish is commonly used in circuit boards, automotive parts or the defense industry. The addition of copper to a part, before the final metal is deposited, can also improve the overall aesthetics of the finished part.
If a single metal does not provide the properties needed, it is also possible to co-deposit two or more metals for an electroplated alloy deposit. One example of this is a copper/tin/zinc alloy, also known as Tri-Metal or Tri-M3, offered by the specialty plating company Electro-Spec, Inc.
**
Finding the Right Electroplating Company for Your Needs**
When looking for a plating company, there are many criteria to consider based on what your project requires and the plating company’s capabilities, including:
- Size of the components
- Volume of pieces (from prototype to volume production)
- Plating metal to be used for the desired outcome
- Budget of the project
- Compliance to industry standards
- Laboratory and testing capabilities/certifications
The method of electroplating is another consideration, as not all facilities necessarily offer the same processes.
“Barrel” plating can efficiently plate large and small volumes of parts where adequate solution exchange and turnover is critical to meeting thickness requirements. Current density within the load of parts in the barrel is typically optimized through the part-to-part contact during rotation.
However, there are some types of parts that are not ideal for most conventional barrels. For example, parts that could become scratched, dented or dinged from part-to-part contact are much more susceptible to damage in most types of barrels. Conversely, some flat parts are not ideal in a barrel due to the parts sticking together during processing, which leads to missing plating or uneven plating thicknesses.
Depending upon some part geometries and tolerances, parts are also more prone to “nesting” into each other during barrel plating.

Vibratory plating, used for small or fragile parts. (Photo courtesy of Electro-Spec, Inc.)
Vibratory plating is used for smaller parts that have deep internal diameters, counter bores, fragile tips/ends or parts that could bend through barrel plating. By incorporating a vibrating or pulsating basket that transfers kinetic energy to the load, the parts move in a clockwise fashion across button contacts on the bottom of the basket. These contacts transfer current to the load of parts and provide very consistent amperage during processing.
Larger parts that have excessive weight or parts that can become entangled or easily nest together cannot be plated in a vibratory basket as they will not move in a uniform fashion. Conversely, smaller parts that do not have enough weight cannot be plated in a vibratory basket, either.

Rack plating is suitable for both delicate and larger parts. It works by holding parts in a fixed position on a rack frame while suspended in the solution. This prevents damage to parts during processing and facilitates the processing of much larger parts that could not be barrel plated.
The biggest issue with rack plating is that direct connection to the parts on the rack causes plating distribution efficiency to be compromised due to high and low current density areas throughout the parts and the rack. Parts placed on a rack also have poor solution movement, which is necessary to control thicknesses, and they are more prone to rinsing and drying stains.
Selective plating processes isolate the plating finish to a select area of the part. This process is done through controlled depth plating, which involves fixing the part in a manner that provides continuous electrical contact and submerges the area to be plated at defined depths through the plating solution.
Selective plating is ideal for specific applications where functional plating is required for performance and/or cost savings with precious metal by reducing the surface area necessary to plate.
While this is an effective method for plating singulated parts and reducing costs, it does require the expense of tooling and labor for loading the parts. There are also some limitations on part size and geometry that can preclude some parts from being able to be selectively plated.

Selective plating of individual parts. (Photo courtesy of Electro-Spec, Inc.)
Spouted Bed Electrode (SBE) plating is designed for small parts, flat parts, parts with counter bores, parts with tabs, parts that nest or have a difficult geometry that make either vibratory plating or conventional barrel plating impossible or impractical.
The SBE process is accomplished in a chamber with ultrasonic action, and continuous solution being pumped into and out of the chamber to facilitate part movement and supply fresh plating electrolyte during the plating process. The SBE ensures very uniform plating coverage in high and low current density areas of the part as well as in counter bores.
The only limitation to SBE is the size of the parts, as the SBE chambers can only accommodate specific sizes and weights that allow for movement inside the chambers.
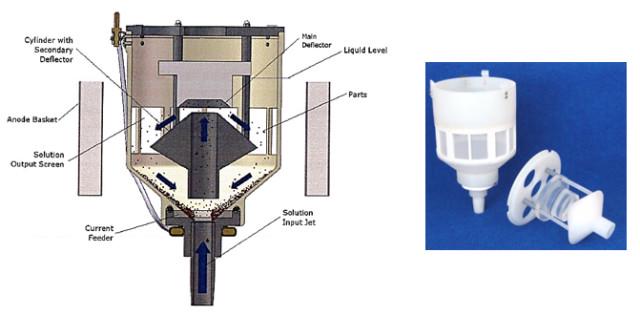
Spouted Bed Electrode plating. (Photo courtesy of George Hradil/Technic, Inc.)
Electro-Spec, Inc., is one example of a specialty plating facility providing high quality and high reliability gold, silver, nickel (electrolytic and electroless), copper and Tri-M3 (tri-alloy) electroplating, as well as passivating, heat treating/ annealing and quality inspection services.
Electro-Spec has also developed, with their supplier, a revolutionary post-plate immersion process called SAMs, or Self Assembled Molecules, that offers enhanced corrosion and contact resistance as well as excellent solderability, while reducing precious metal use and cost. SAMs is a surface treatment that forms a protective layer on gold, silver, Tri-M3 and other plated metals.
For more information on Electro-Spec, Inc., check out their website here, or their video below.
1 Electroplating Basic Principles, Processes and Practice by Nasser Kanani. Atotech Deutschland GmbH Berlin, Germany; Published by Elsevier Kidlington, Oxford 2004
Electro-Spec, Inc. has sponsored this post. It has no editorial input to this post. All opinions are mine. –Meghan Brown





