Surfaces are the silent enablers of contamination. From surgical clamps to food-grade valves, the materials we trust in sterile environments often fall short under microbial pressure. Pathogens colonize quickly, traditional coatings degrade, and sterilization protocols can only go so far.
As electronic systems become smaller, faster, and even more heavily relied upon, high-frequency performance has become a critical design requirement—especially in telecommunications, aerospace, and medical devices. RF connectors, in particular, must maintain signal integrity under increasingly demanding conditions.
Rework and scrap pose critical challenges to manufacturers, particularly in industries requiring precision and reliability. Defective or out-of-spec components lead to increased costs, production delays, and strained supplier relationships. For safety-critical components in highly regulated industries like aerospace and automotive, these issues are especially damaging, creating bottlenecks that disrupt the supply chain and delay deliveries to OEMs.
Know your process and requirements for durable, high conductivity, corrosion resistant plating.
A wide range of devices with numerous applications use precious metal plating. This process, however, can often be expensive and the precious metal is subjected to wear and tear that can eventually cause failures. Electro-Spec’s innovative methods for plating include a technological advancement known as NanoSHIELD-AU. The NanoSHIELD-AU process allows, for example a gold plated component to have a much thinner deposit without a reduction in functionality.
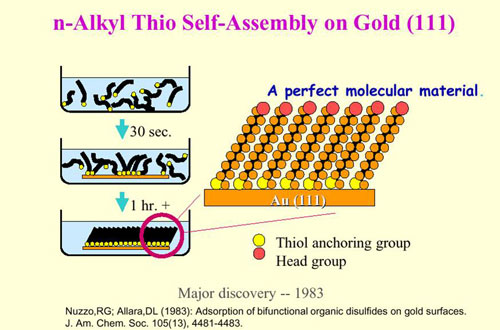
What is NanoSHIELD-AU?
SAM is an acronym for Self-Assembled Molecules. The NanoSHIELD-AU process employs bi-Functional or multi-functional molecules that offer two or more termination groups with different functionality. Essentially, the NanoSHIELD-AU process uses molecules that offer two levels of protection. The molecules used in the NanoSHIELD-AU method can be attached to metal alloys as well as ceramic, glass, plastics, and more. Right now, NanoSHIELD technology is only being used as a post-plate surface treatment for gold and tri-alloy (Tri-M3).
Electro-Spec. Inc. is proud to announce Volume II of technical blogs which will cover several of its most valuable testing and inspection capabilities to meet a broad range of industry standards and customer requirements. This volume will highlight five different types of testing that allow Electro-Spec to distinguish itself as a world class electroplating facility. Each testing method will be fully explained which should allow customers (present and future) to better understand the results of the various tests. Electro-Spec will always place meeting or exceeding plating requirements via test methods as a main priority and thus resulting in the highest quality surface finish possible.
Electro-Spec Inc. is more than capable of handling all of your precious metal plating and passivation needs for the medical industry including medical devices, instrumentation, and electrical connectors. With state-of-the art equipment, processes and technology, Electro-Spec will provide quality which is unparalleled by any other plating facility. We have the ability to meet the most stringent specifications in the medical industry, with indefinite traceability for all processes that we do. The most common metals utilized to plate medical components are gold and silver; these are metals which Electro-Spec has the capabilities of using. In addition, Electro-Spec provides passivation services which have been proven to allow medical instrumentation to have a more sterile surface.
The Medical, Automotive, Aerospace, Military and RF/Microwave Industries, regardless of their unique functions, often demand exceptional conductivity. Electro-Spec intends to meet the demands which sophisticated electrical components require.
Electroplating is a process whereby one metal is plated onto another via an electrodeposition method. Customers seek out electroplating for their parts for many reasons such as aesthetics, corrosion protection, increased hardness, wear resistance, increased conductivity, and decreased friction. It allows manufacturers to use base metals that are less expensive and apply a high quality coating to them to achieve the certain desired properties on the finished part.
Electro-Spec has been providing award winning electroplating and electroless plating services to customers for over five decades. This includes applications for lifesaving and safety critical components. Plating is available in precious and semi-precious materials including gold, silver, nickel, copper, Tri-M3TM (Tri-Alloy), electroless and electrolytic nickel. This short article discusses these plating options, as well as their benefits and examples of industries that they are often found of use within.